César González (UCM) — Dissociation of water molecules over defective 2D materials for hydrogen production
Don't miss any Success Story following us on X and LinkedIn!
@RES_HPC RES - Red Española de Supercomputación
Check this Success Story at our LinkedIn: Dissociation of water molecules over defective 2D materials for hydrogen production
💡The first Success Story of the year is about potential sustainable hydrogen production💡
📋 "Dissociation of water molecules over defective 2D materials for hydrogen production” led by César González Pascual from Universidad Complutense de Madrid
Molybdenum disulphide (MoS2) is a promising alternative to expensive precious materials currently used in water electrolysis. This abundant material could revolutionize clean hydrogen energy is produced.
Using advanced Density Functional Theory calculations, the team created a massive 340-atom cluster to study how water molecules break down at different sites in the material. They compared reactions at sulfur vacancies and material edges, discovering that Mo-edges are significantly more effective at breaking water molecules.
🖥️ Thanks to RES supercomputing power, they could simulate multiple atomic configurations and identify the most efficient pathways for hydrogen production. Their findings show that Mo-edges are 0.43 eV more effective than sulfur vacancies for water dissociation.
These findings are crucial for developing better catalysts for hydrogen production, potentially making clean energy more accessible and affordable, and paves the way for further studies on the topic. Particularly, the team will study the energy barriers and the effect of Niobium doping in future works.
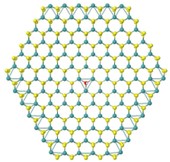
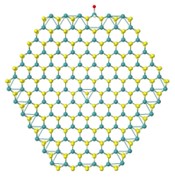
📸 The left image shows a H2O molecule dissociated on the Mo-edge resulting a Mo atom bonded to the O, while a H2 molecule remains close to this site. In the right image, the H2O molecule is dissociated in the S-vacancy where the O atom occupies the original S-site while the H2 molecule stays above physically (and not chemically) adsorption.
📋 "Dissociation of water molecules over defective 2D materials for hydrogen production” led by César González Pascual from Universidad Complutense de Madrid
Molybdenum disulphide (MoS2) is a promising alternative to expensive precious materials currently used in water electrolysis. This abundant material could revolutionize clean hydrogen energy is produced.
Using advanced Density Functional Theory calculations, the team created a massive 340-atom cluster to study how water molecules break down at different sites in the material. They compared reactions at sulfur vacancies and material edges, discovering that Mo-edges are significantly more effective at breaking water molecules.
🖥️ Thanks to RES supercomputing power, they could simulate multiple atomic configurations and identify the most efficient pathways for hydrogen production. Their findings show that Mo-edges are 0.43 eV more effective than sulfur vacancies for water dissociation.
These findings are crucial for developing better catalysts for hydrogen production, potentially making clean energy more accessible and affordable, and paves the way for further studies on the topic. Particularly, the team will study the energy barriers and the effect of Niobium doping in future works.
📸 The left image shows a H2O molecule dissociated on the Mo-edge resulting a Mo atom bonded to the O, while a H2 molecule remains close to this site. In the right image, the H2O molecule is dissociated in the S-vacancy where the O atom occupies the original S-site while the H2 molecule stays above physically (and not chemically) adsorption.



