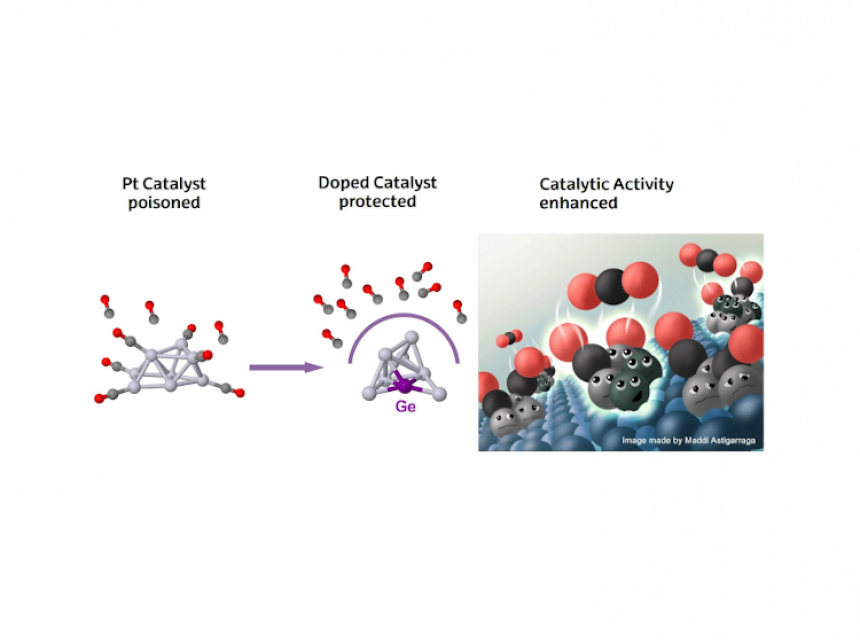
Elisa Jiménez/Txema Mercero (UPV/EHU) — Fight the CO poisoning on the catalysts to improve the performance in different chemical environments
Don't miss any Success Story following us on X and LinkedIn!
@RES_HPC RES - Red Española de Supercomputación @res-icts.bsky.social
Check this Success Story at our LinkedIn: Fight the CO poisoning on the catalysts to improve the performance in different chemical environments
💡A RES Success Story about overcoming CO poisoning in Pt catalysts💡
📋 "Fight the CO poisoning on the catalysts to improve the performance in different chemical environments", part of Andoni Ugartemendia's thesis and led by Elisa Jimenez and J.M. Mercero from UPV/EHU - Donostia International Physics Center (DIPC)
CO poisoning consists in the strong binding of CO to catalyst active sites, blocking them and reducing the efficiency of catalytic reactions. However, this can be overcome with Pt-based nanocatalysts alloyed with Ge and using 2D supports.
🖥️ Thanks to RES supercomputer hashtagMareNostrum5, the team investigated how composition, size, and support interactions influence catalytic behaviour using global optimization techniques, electronic structure analysis, and evaluated the catalytic activity.
The research yielded the following conclusions:
🔹Ge doping conferes Pt clusters remarkable CO poisoning resistance.
🔹The catalytic performance strongly depends on Ge content.
🔹 For CO oxidation, alloying Pt clusters with Ge creates a bifunctional system with distinct active sites (Pt for CO, Ge for O₂), avoiding competition or overbinding
🔹When deposited on 2D surfaces, metal-support interactions stabilize Pt and PtGe clusters and enable to tune their electronic structure and catalytic performance for hydrogen oxidation reaction (HOR). Combined with Ge alloying, it even makes CO adsorption unfavorable in systems like Pt₅Ge₅
Additionally, they obtained powerful and potential implications of these findings:
🔹Ge is revealed as a powerful alloying agent to improve the selectivity and sintering resistance of Pt-based nanocatalysts.
🔹The electronic properties of pure and doped Pt catalysts can be tailored through size, composition and support, offering a powerful way to fine-tune catalytic behavior for target reactions.
🔹While oxophilic sites hinder oxygen reduction activity in acidic media, the combination of Ge alloying and 2D support materials shows excellent potential for improving hydrogen oxidation reaction (HOR) performance.
📷 Illustrative image made by Maddi Astigarraga Bergara
📋 "Fight the CO poisoning on the catalysts to improve the performance in different chemical environments", part of Andoni Ugartemendia's thesis and led by Elisa Jimenez and J.M. Mercero from UPV/EHU - Donostia International Physics Center (DIPC)
CO poisoning consists in the strong binding of CO to catalyst active sites, blocking them and reducing the efficiency of catalytic reactions. However, this can be overcome with Pt-based nanocatalysts alloyed with Ge and using 2D supports.
🖥️ Thanks to RES supercomputer hashtagMareNostrum5, the team investigated how composition, size, and support interactions influence catalytic behaviour using global optimization techniques, electronic structure analysis, and evaluated the catalytic activity.
The research yielded the following conclusions:
🔹Ge doping conferes Pt clusters remarkable CO poisoning resistance.
🔹The catalytic performance strongly depends on Ge content.
🔹 For CO oxidation, alloying Pt clusters with Ge creates a bifunctional system with distinct active sites (Pt for CO, Ge for O₂), avoiding competition or overbinding
🔹When deposited on 2D surfaces, metal-support interactions stabilize Pt and PtGe clusters and enable to tune their electronic structure and catalytic performance for hydrogen oxidation reaction (HOR). Combined with Ge alloying, it even makes CO adsorption unfavorable in systems like Pt₅Ge₅
Additionally, they obtained powerful and potential implications of these findings:
🔹Ge is revealed as a powerful alloying agent to improve the selectivity and sintering resistance of Pt-based nanocatalysts.
🔹The electronic properties of pure and doped Pt catalysts can be tailored through size, composition and support, offering a powerful way to fine-tune catalytic behavior for target reactions.
🔹While oxophilic sites hinder oxygen reduction activity in acidic media, the combination of Ge alloying and 2D support materials shows excellent potential for improving hydrogen oxidation reaction (HOR) performance.
📷 Illustrative image made by Maddi Astigarraga Bergara